Drug Development Process
The development of drugs involves a meticulous and coordinated process in which lead molecules are transformed into safer and more effective medicines. During this process, new medicines are ensured to comply with high standards and contribute to improved health outcomes through collaboration among researchers, practitioners, regulators and industry professionals. In the process of developing medicines, a number of stages are necessary to bring new medicinal products out of concept and into the market:-
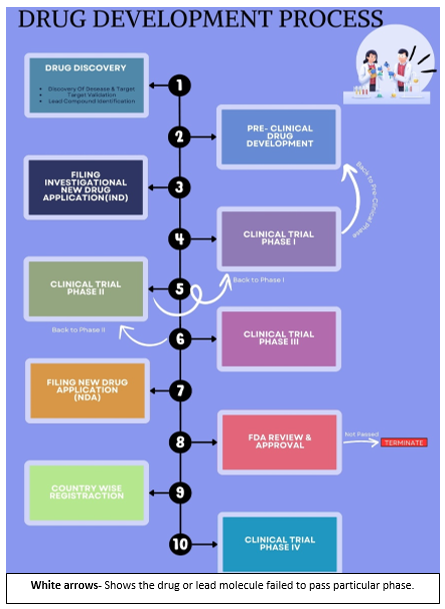
1. Drug Discovery and Research
- Target identification:-
Identifying a potential target for drug development, often a specific molecule or biological pathway that is associated with certain diseases, is the first stage of this process.
- Using a variety of instruments and techniques, including high-throughput screening as well as computing methods, researchers carry out large studies in order to gain an understanding of the objective.
- Target validation:- When you have selected a possible target, you have to demonstrate that the target is involved in the process of progression of a particular disease and that the activity of the target molecule can be easily regulated.
- Identification of Lead Compound:- This process involves identification of a compound which can interact with the selected target. Different screening experiments can be done to select the natural compound which can be re-purposed like drugs. Synthetic compounds can also be designed that can target the potential target and not interfere in other processes that takes place in the cells
- Lead Optimization:- Once lead compounds are found, they're optimized for safety and effectiveness. Modifications are made to prevent them from binding to unintended targets. Dosage and administration routes are tested on cell cultures before safety assessments in animals.
2. Preclinical Studies:
- In order to assess safety, efficacy and potential adverse reactions, a promising compound is subjected to preclinical testing in laboratory and animal models when it has been identified.
- In order to inform the design of subsequent clinical studies, researchers produce valuable data on pharmacokinetics, pharmacodynamics and toxicity.
3. Investigational New Drug Application (IND):
- Before moving to human trials, researchers submit an IND application to regulatory authorities, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), providing comprehensive data from preclinical studies.
- The initiation of clinical trials is facilitated by the approval of an Investigational New Drug application.
4. Clinical proceedings:
- In order to evaluate safety, dosage, efficacy and side effects of the medicinal product in human subjects, this stage consists of three stages: Phase I, II and III clinical studies.
(a) Phase I Clinical Trial- The initial phase of trials, involves less people generally <15. Phase I trial evaluates the safety and efficacy of new drug in healthy volunteers. Among all, approximately 70% drugs advance to phase II.
- Determines a drug's toxicity, absorption, distribution, metabolism and excretion.
(b) Phase II Clinical Trial- Phase II trials checks safety and efficacy. Participants usually have the targeted health conditions. It involves several hundred participants (200-500) who already have the condition the medication is designed to treat.
- If Phase II results are promising, the medication may advance to Phase III trials.
(c) Phase III Clinical Trial- It is final trial in which new medication is compared with existing treatment for some conditions. It involves large group, nearly 3000-5000 participants
- Results usually provide package insert info (Prescribing Information for doctors, and Patient Information materials)
5. New Drug Application (NDA) Submission:
- The successful completion of clinical studies allows the submission of a New Drug Application to FDA for marketing authorization.
- All the information related to the drug, including all studies, data of preclinical and clinical studies, interactions with other drugs, precautions, and side effects is provided.
- In order to ensure the safety, efficacy and quality of the medicinal product before it is given marketing authorization, the NDA shall be reviewed by regulators.
6. FDA review:
When results from the three phases of clinical trials are obtained and the drug has been appropriately formulated for a good safety and efficacy, it is forwarded for being reviewed by FDA (Food and Drug Administration).
7. Country-wise Registration:
When a drug is approved from USFDA, you have to register it in those countries where you want to sell it. All countries have different guidelines for registering drugs.
8. Post-Marketing Surveillance:
- Phase 4 trials aim to evaluate the drug’s performance in real-life scenarios.
- After approval, it is essential to continue monitoring the safety and efficacy of the medicinal product for any unusual or long term adverse reactions which may not be detected in human studies.
Any previously missed uncommon or long-term effects can be detected.
Regulatory guidelines for drug development:
There are strict laws and regulations governing the drug development process. The Basic Law of the United States is a Federal Food, Drug and Cosmetic Act to ensure that medicines are safe and effective.
The process of drug development in India is governed by the new rules on drugs and clinical trials, published in 2019.

