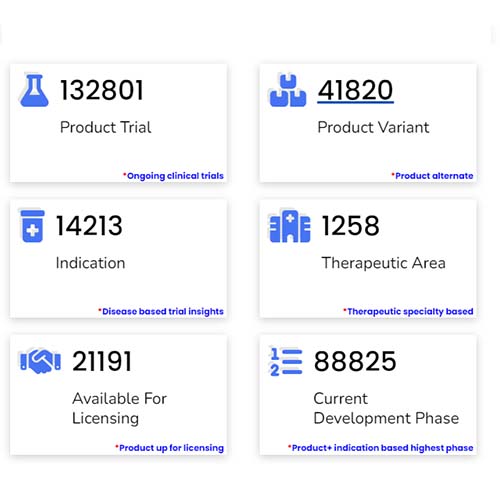
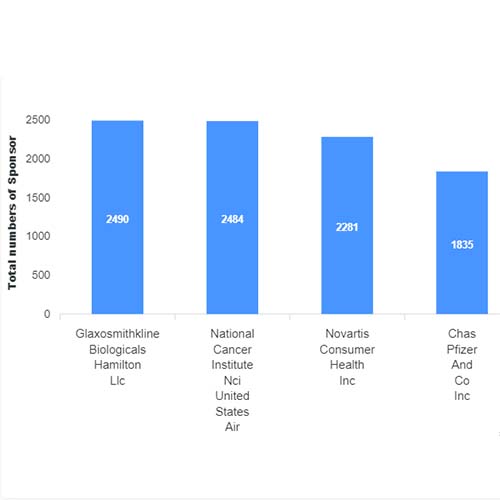
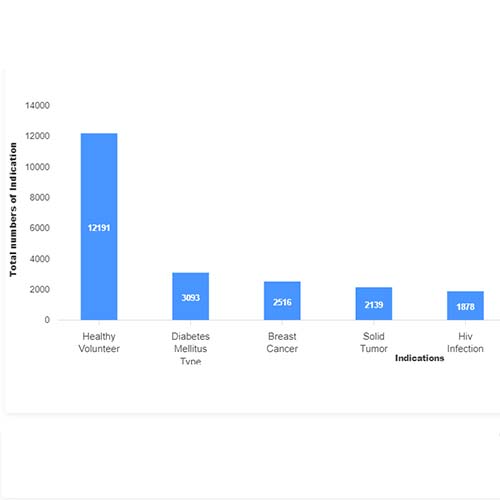
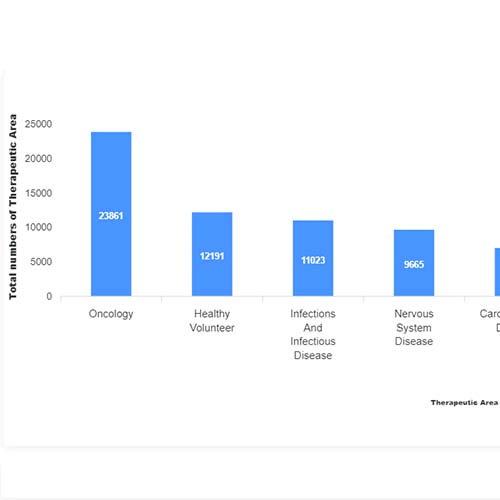
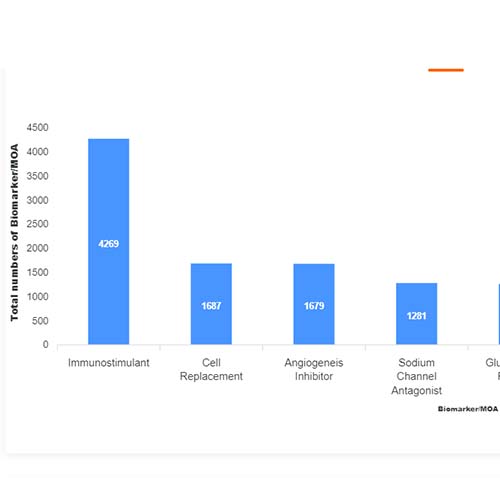
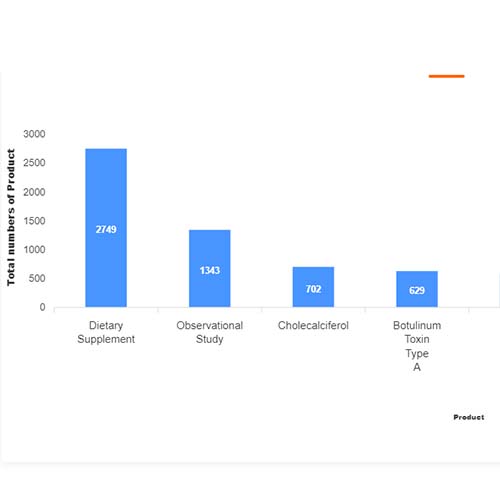
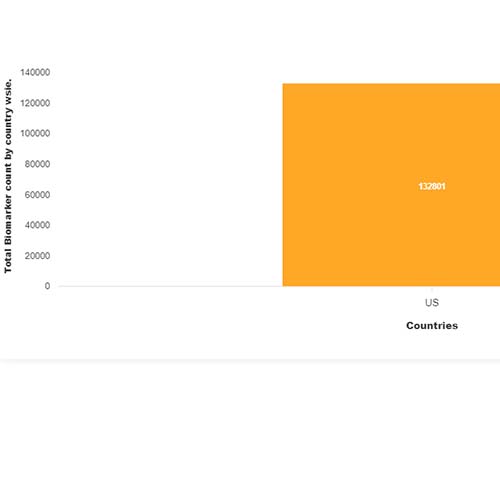
Basic Analysis
• It involves Product variant, product trial, product type, MOA/biomarker, indication, therapeutic area, sponsor, approved, available for licensing, current development phase, patent expiry, and global historical sales data.
• Includes only unique trials and simultaneously excludes terminated, withdrawn, and trials of unknown status.