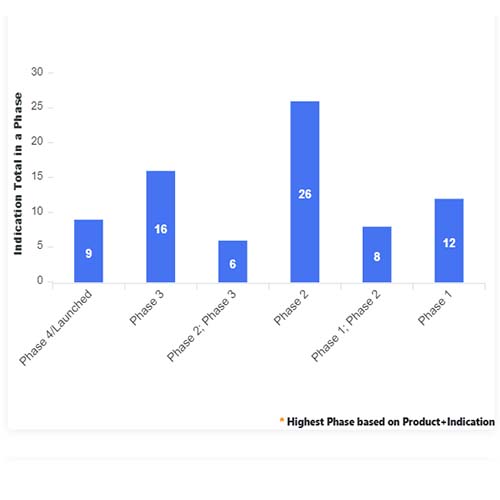
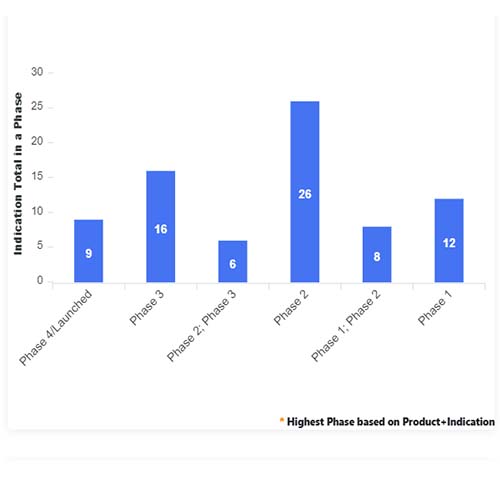
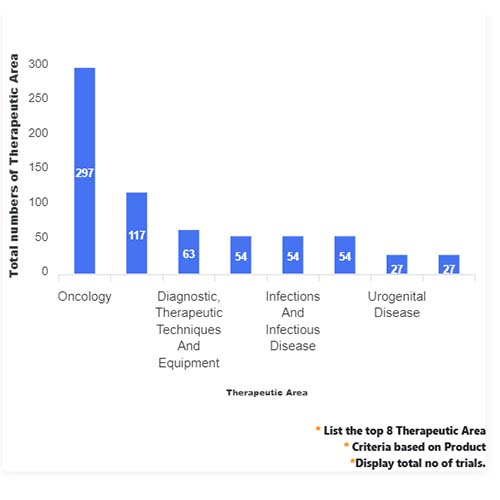
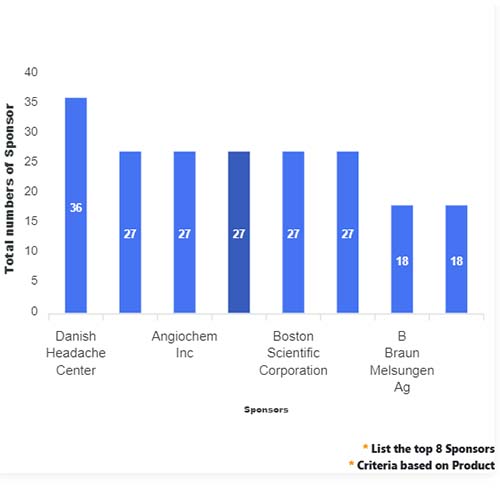
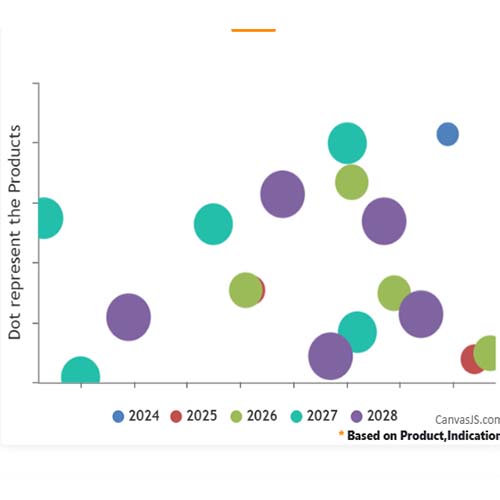
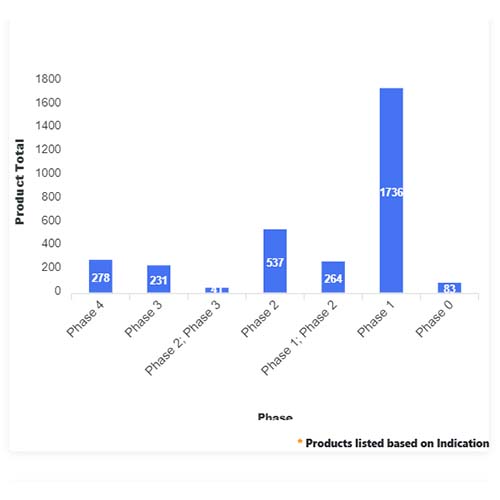
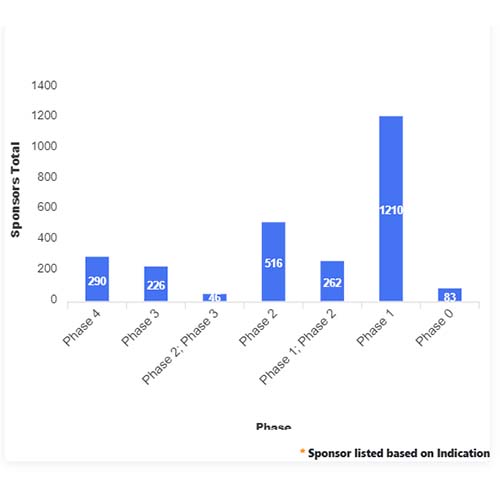
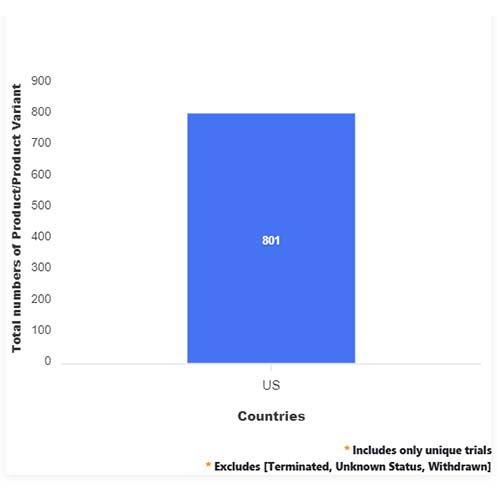
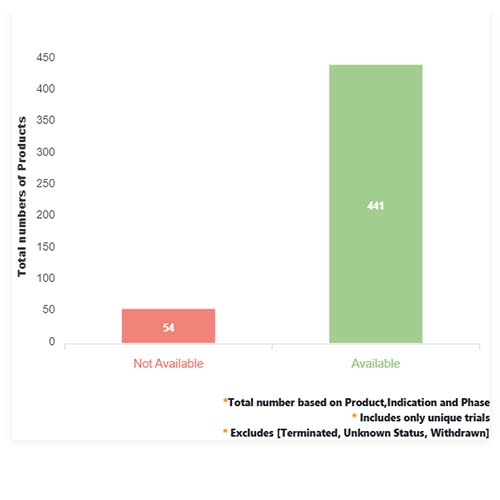
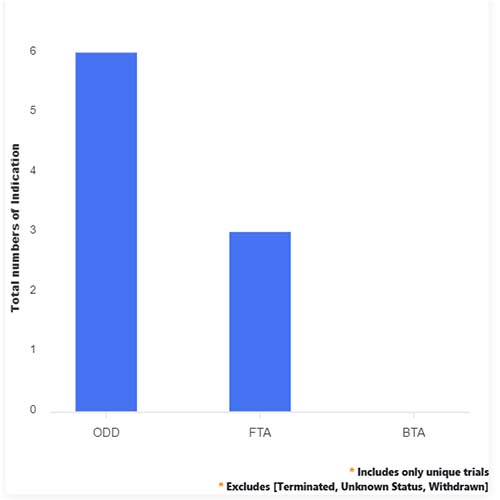
Basic Analysis
• In the basic analysis section, we explore product variants, product trials, product types, target/biomarker, therapeutic area, sponsors, and indications.
• We only focus on unique trials excluding terminated, withdrawn, and trials of unknown status.