Ascletis Begins Dosing in U.S. Phase IIa Trial of...
Category : Clinical Trial Update 4 July 2025Overview Ascletis Pharma Inc. (HKEX: 1672, ""Ascletis"") announces today that...
Newsletter- All News is a comprehensive section of updated news and current trends, specifically curated to keep clinical trials, including breakthroughs in drug research, regulatory approvals, and advancements in study methodologies. Our news covers key trends in trial designs, patient recruitment strategies, and cutting-edge technologies shaping the future of clinical research

Overview Ascletis Pharma Inc. (HKEX: 1672, ""Ascletis"") announces today that...
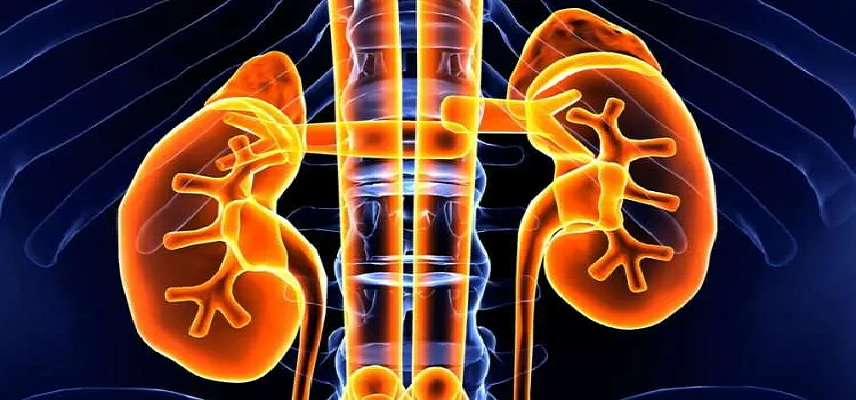
Overview Everest Medicines a biopharmaceutical company focused on the discovery, clinical develop...

Overview Gain Therapeutics, Inc, a clinical-stage biotechnology company leading the discovery and...

Novartis today announced top-line results from the Phase III GCAptAIN study evaluating Cosentyx®...

Overview Moderna, Inc, a leader in the creation of the field of mRNA medicine, announced positive...