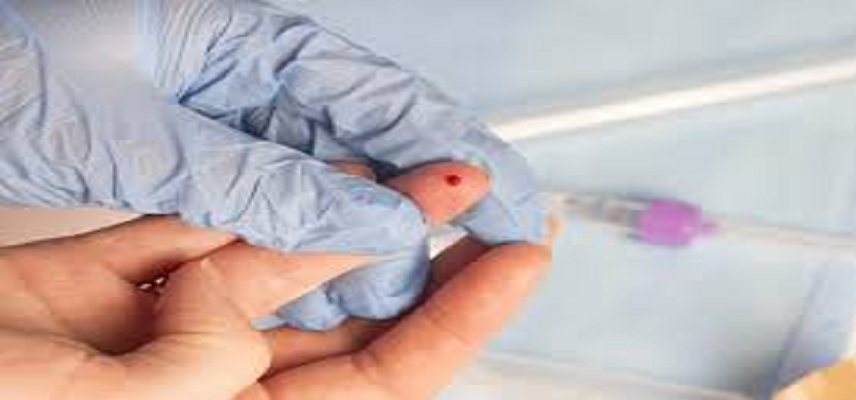
Finger-Prick Blood Test Could Transform Alzheimer’s Diagnosis
A major international research effort is testing whether a simple finger-prick blood test can help diagnose Alzheimer’s disease earlier, faster, and at lower cost.
If successful, it could replace or reduce reliance on expensive brain scans and invasive spinal taps—methods that remain inaccessible for many patients worldwide.
Why Alzheimer’s Diagnosis Needs a Reset
Today, Alzheimer’s is typically diagnosed using:
- PET scans
- MRI scans
- Lumbar punctures (spinal taps)
These methods are:
- Expensive
- Invasive
- Time-consuming
- Limited to specialized healthcare centers
As disease-modifying therapies emerge, this diagnostic bottleneck is becoming a critical problem.
How the Finger-Prick Test Works?
The test uses a dried blood plasma separation card, collected via a finger prick.
Key advantages:
- No refrigeration required
- Stable at room temperature
- Easy to store and ship
- Low-cost and scalable
This makes it suitable for large population screening and use in low-resource settings.
What the Study Is Measuring?
Researchers are analyzing three blood-based biomarkers strongly linked to Alzheimer’s disease:
- pTau217 (phosphorylated tau 217)
- GFAP (glial fibrillary acidic protein)
- NfL (neurofilament light polypeptide)
These results will be compared against:
- PET and MRI scans
- Other blood-based tests
- Digital biomarkers such as speech analysis, retinal imaging, and cognitive testing
Inside the Bio-Hermes-002 Trial
The finger-prick test is being evaluated within the Bio-Hermes-002 study, led by the Global Alzheimer’s Platform Foundation (GAP).
Key facts:
- Target enrollment: 1,000 participants
- Enrolled so far: 883
- Study sites: 25 across the UK, US, and Canada
- Participants include:
- Cognitively normal individuals
- People with mild cognitive impairment
- Patients with mild to moderate Alzheimer’s
More than 360 participants have already completed the finger-prick test.
Partners include:
- LifeArc
- UK Dementia Research Institute (UK DRI) and its Biomarker Factory
Why Early Detection Changes Everything?
Earlier diagnosis means:
- Earlier access to emerging disease-modifying therapies
- Better chances of slowing disease progression
- Improved patient planning and support
According to LifeArc, blood-based biomarkers now make it possible to identify high-risk individuals before symptoms appear, but only if testing is affordable and accessible.
Expert Perspectives
LifeArc emphasizes scalability and access:
- Cheaper tests enable wider screening
- More patients can qualify for early-stage treatments
GAP highlights equity:
- Simple blood tests could reach people without access to specialist clinics
UK DRI researchers underline the scientific value:
- Results will be directly benchmarked against gold-standard diagnostics
- The study includes a high proportion of under-represented populations
A Personal Story Behind the Science
Dr Michael Sandberg, a London-based GP, joined the study after witnessing Alzheimer’s firsthand through his mother’s diagnosis.
Key takeaways from his experience:
- Clinical trials gave his mother meaningful extra time
- Early detection can change treatment trajectories
- A negative test result brought personal relief
For clinicians like him, accessible screening is essential if new treatments are to reach the people who need them most.
Addressing the Diversity Gap in Alzheimer’s Research
The study is designed to recruit at least 25–30% of participants from under-represented ethnic groups. This matters because:
- Black and Hispanic populations have up to 2x higher Alzheimer’s risk
- Clinical trial participation rates often remain below 20%
- Personalized medicine depends on representative data
Without diversity, future diagnostics and treatments risk widening health inequities.
What Comes Next?
More evidence is needed before a finger-prick Alzheimer’s test can be adopted by national health systems like the NHS.
But Bio-Hermes-002 aims to deliver:
- Large-scale validation
- Internationally diverse data
- A practical alternative to invasive diagnostics
If successful, this approach could redefine how Alzheimer’s is detected worldwide—moving diagnosis earlier, closer to patients, and into everyday clinical practice.
Bottom line
A finger-prick blood test won’t cure Alzheimer’s.
But it could unlock early diagnosis at scale—and that may be the most powerful tool we have right now.

Optimize Your trial insights with Clival Database.
Are you exhausted from the uncertainty of trial insights pricing? Clival Database ensures the clarity in the midst of the global scenario for clinical trials to you.Clival Database is one of the best databases that offers an outstanding number of clinical trial data in terms of 50,000+ molecules and from primary regulatory markets as well as new entrants like Indian and Chinese markets.
With Clival, you get accurate positioning of historical sales data, patent database, company profiling, safety & efficacy, and prediction of launch of new innovative molecules helping you to align your research and driving down the cost.
To add value, we further break down our analytics for you so that improving your operational effectiveness; optimizing your clinical trials; and offering you accurate and high-quality data at lowest possible prices becomes possible.
Elevate your trial success rate with the cutting-edge insights from Clival database.
Check it out today and make more informed sourcing decisions! Learn More!