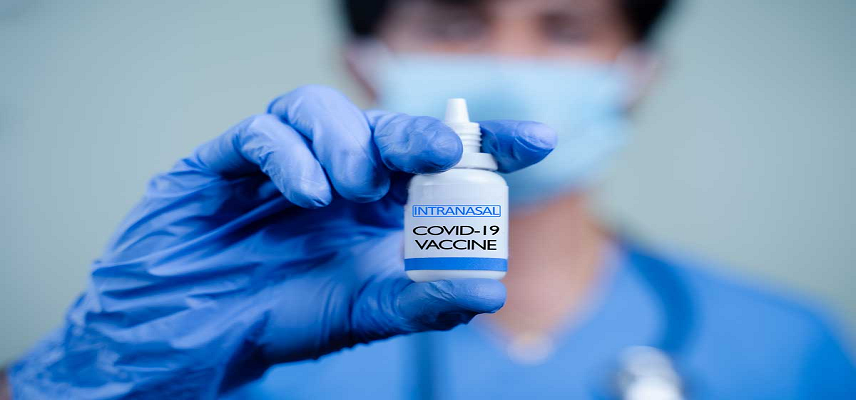
Ocugen Announces FDA Approval of OCU500 Inhaled Vaccine for COVID-19
Ocugen has received confirmation from the U.S. Food and Drug Administration (FDA) that its Investigational New Drug (IND) application for OCU500, an inhaled mucosal vaccine for COVID-19, is now in effect.
OCU500 uses a chimpanzee adenovirus-vectored (ChAd36) technology, which has shown promise in previous studies.
Ocugen plans to extend this platform to other respiratory viruses, such as seasonal flu, bird flu, and respiratory syncytial virus (RSV).
This approval marks a key milestone for the company as it prepares to initiate a Phase 1 clinical trial.
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, will sponsor and manage the trial, which aims to evaluate the safety, tolerability, and immune response of OCU500 delivered through two methods: inhalation into the lungs and intranasal spray.
According to the Centers for Disease Control (CDC), between October 2024 and January 2025, there were between 4.4 and 7.9 million COVID infections, leading to 120,000 to 210,000 hospitalisations and 14,000 to 25,000 deaths.
The Phase 1 trial will enrol 80 adults aged 18 to 64 years, split into low-dose and high-dose groups, with each group receiving either the inhaled or intranasal form of the vaccine.
The primary focus of the trial is safety, while secondary objectives include measuring antibody production and tracking the incidence of breakthrough COVID-19 infections.
The ChAd36 vector technology was originally developed and licensed from Washington University in St. Louis.
Project NextGen, a $5 billion initiative involving multiple government agencies, aims to accelerate the development of vaccines and therapeutics for COVID-19.
Ocugen has also advanced the ChAd36 vector technology, enabling it to carry multiple antigens in one vector.
This innovation offers significant flexibility in vaccine production, enabling rapid responses to emerging variants of concern.

Optimize Your trial insights with Clival Database.
Are you exhausted from the uncertainty of trial insights pricing? Clival Database ensures the clarity in the midst of the global scenario for clinical trials to you.Clival Database is one of the best databases that offers an outstanding number of clinical trial data in terms of 50,000+ molecules and from primary regulatory markets as well as new entrants like Indian and Chinese markets.
With Clival, you get accurate positioning of historical sales data, patent database, company profiling, safety & efficacy, and prediction of launch of new innovative molecules helping you to align your research and driving down the cost.
To add value, we further break down our analytics for you so that improving your operational effectiveness; optimizing your clinical trials; and offering you accurate and high-quality data at lowest possible prices becomes possible.
Elevate your trial success rate with the cutting-edge insights from Clival database.
Check it out today and make more informed sourcing decisions! Learn More!