Oscotec & ADEL Begins Trial for ADEL-Y01 in Alzheimer's Disease
Oscotec/ADEL Initiates First-in-Human Dosing in Ph1 of Anti-MTBR Tau Antibody ADEL-Y01 In Alzheimer's Disease
Oscotec Inc. and ADEL Inc. announced the dosing of the first healthy participant in its first-in-human study of ADEL-Y01 for the treatment of Alzheimer's disease (AD).
Oscotec and ADEL are jointly developing a novel disease-modifying immunotherapy agent (ADEL-Y01) targeting tau protein accumulation in the AD brain. ADEL-Y01 is uniquely designed to target tau protein acetylated at lysine-280 (acK280), inhibiting aggregation and propagation of toxic tau and thus may slow the course of the disease.
Phase I Trial
This phase 1a/1b study in the U.S. is to evaluate the safety, tolerability, pharmacokinetics, and clinical activity of ADEL-Y01 in 40 healthy volunteers as well as in 33 participants with Mild Cognitive Impairment due to Alzheimer's disease or mild Alzheimer's disease.
The Target: Tau
"Given that tau is strongly correlated with AD progression and clinical severity, targeting of tau is anticipated to effectively slow the disease progress. As our understanding of the pivotal role of the tau mid-region MTBR increases, selecting the right epitope becomes crucial in developing tau-targeting therapeutics to attain clinical benefits", explained SeungYong Yoon, MD/PhD, CEO of ADEL.
Words from Oscotec
Oscotec is pleased to reach a significant milestone leading the clinical study as a co-developer with ADEL, and we will continue to bring innovative medicines in neurogenerative diseases"", said Taeyoung Yoon, CEO of Oscotec.
Preclinical Results
- The preclinical results of ADEL-Y01 in animal models were published last year, demonstrating improvement in memory impairment, behavioral deficits through the inhibition of Tau seeding and propagation.
- ("Monoclonal antibody Y01 prevents tauopathy progression induced by lysine 280–acetylated tau in cell and mouse models" in The Journal of Clinical Investigation 2023)
About Oscotec Inc
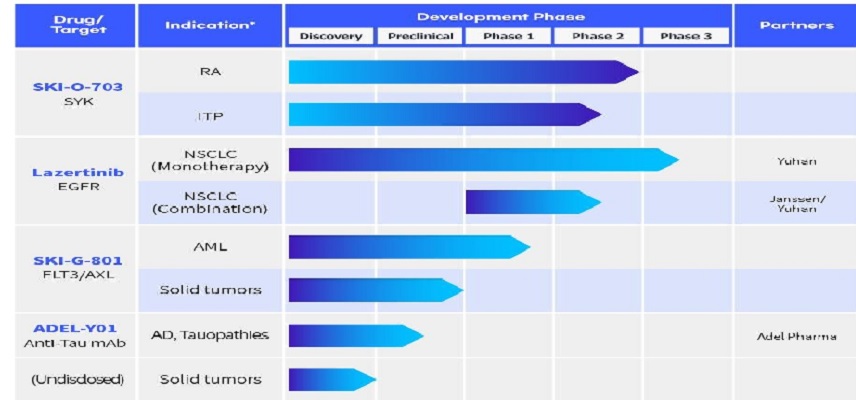
Oscotec is a clinical stage drug discovery and development company pursuing translation of rigorous science into innovative medicine for clinically unmet needs.. The Company's clinical pipeline consists of targeted therapeutics in immunology and oncology. Oscotec is developing cevidoplenib (SYK inhibitor) for ITP (Phase 2, completed) and FLT3/AXL inhibitor for AML (Phase 1) as well as solid tumors (Phase 1). The company is also the originator of Lazertinib (LECLAZA®), a 3rd generation EGFR inhibitor being developed by Janssen Pharma and Yuhan Corp. for lung cancers.
About ADEL Inc
ADEL is a leading company of cutting-edge technologies for the treatment and diagnosis of neurological diseases including Alzheimer's disease. Founded by scientists who have investigated Alzheimer's disease at Asan Medical Center, University of Ulsan College of Medicine, Korea, ADEL is developing smart, innovative drug candidates for the patients worldwide.

Optimize Your trial insights with Clival Database.
Are you exhausted from the uncertainty of trial insights pricing? Clival Database ensures the clarity in the midst of the global scenario for clinical trials to you.Clival Database is one of the best databases that offers an outstanding number of clinical trial data in terms of 50,000+ molecules and from primary regulatory markets as well as new entrants like Indian and Chinese markets.
With Clival, you get accurate positioning of historical sales data, patent database, company profiling, safety & efficacy, and prediction of launch of new innovative molecules helping you to align your research and driving down the cost.
To add value, we further break down our analytics for you so that improving your operational effectiveness; optimizing your clinical trials; and offering you accurate and high-quality data at lowest possible prices becomes possible.
Elevate your trial success rate with the cutting-edge insights from Clival database.
Check it out today and make more informed sourcing decisions! Learn More!