Subcutaneous Injection Pen for Methotrexate Gains Approval
Metoject® Subcutaneous Injection Pen for Methotrexate Receives Regulatory Approval as Autoinjector in Japan
Overview
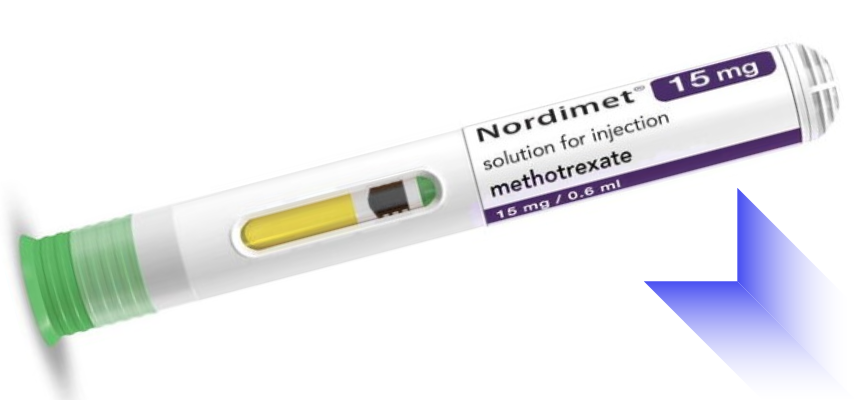
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito) and nippon medac Co., Ltd. (Headquarters: Tokyo, CEO: Hirohisa Iriyama), a subsidiary of medac Gesellschaft für klinische Spezialpräparate mbH (Headquarters: Germany), jointly announced today that they have received manufacturing and marketing approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for an enhanced formulation of Metoject Subcutaneous Injection, an anti-rheumatic agent delivered via a pen-type autoinjector. This formulation, titled ""Metoject® Subcutaneous Injection 7.5mg Pen 0.15mL, 10mg Pen 0.20mL, 12.5mg Pen 0.25mL, and 15mg Pen 0.30mL,"" incorporates the previously approved Metoject Subcutaneous Injection pre-filled syringe into a more convenient pen-type autoinjector.
Aims for Development
The development of this formulation aims to alleviate the burden on patients and enhance safety during self-injection. The pen-type autoinjector allows for easy self-administration in two simple steps: removing the cap and pressing the pen against the skin. Moreover, the built-in needle cover ensures that the needle remains concealed prior to administration and automatically locks after use to prevent accidental skin puncture.
Rheumatoid Arthritis & Methotrexate
Rheumatoid arthritis affects an estimated 700,000 - 800,000 individuals in Japan, with methotrexate being a primary treatment option. Eisai and nippon medac are dedicated to providing this innovative treatment option to alleviate the challenges faced by rheumatoid arthritis patients. They aim to further contribute to meeting the diverse needs of these patients and enhancing the benefits they receive.
Methotrexate
Methotrexate (MTX) serves as a cornerstone drug in the treatment of rheumatoid arthritis. It is believed to regulate cell growth by inhibiting folate metabolism in lymphocytes and other cells, and also exhibits anti-inflammatory effects by promoting adenosine synthesis in vascular endothelial cells and other cells within synovial membranes. The Metoject Subcutaneous Injection Pen represents the first self-administerable MTX subcutaneous injection pen-type autoinjector for rheumatoid arthritis in Japan, with approvals in over 16 countries across Europe.

Optimize Your trial insights with Clival Database.
Are you exhausted from the uncertainty of trial insights pricing? Clival Database ensures the clarity in the midst of the global scenario for clinical trials to you.Clival Database is one of the best databases that offers an outstanding number of clinical trial data in terms of 50,000+ molecules and from primary regulatory markets as well as new entrants like Indian and Chinese markets.
With Clival, you get accurate positioning of historical sales data, patent database, company profiling, safety & efficacy, and prediction of launch of new innovative molecules helping you to align your research and driving down the cost.
To add value, we further break down our analytics for you so that improving your operational effectiveness; optimizing your clinical trials; and offering you accurate and high-quality data at lowest possible prices becomes possible.
Elevate your trial success rate with the cutting-edge insights from Clival database.
Check it out today and make more informed sourcing decisions! Learn More!