ZygoFix’s Zlock Lumbar Facet Fixation System Receives US FDA Clearance
Overview
ZygoFix Ltd, a portfolio company of The Trendlines Group Ltd (Trendlines) announced that it has received regulatory clearance from the United States Food and Drug Administration for its zLOCK Lumbar Facet Fixation System. This achievement was supported by compelling clinical evidence from the company's ongoing European clinical study, marking a significant advancement in spinal fusion technology.
Spinal Fusion
- Spinal fusion, a common surgical procedure performed for degenerative spinal conditions such as spondylolisthesis, spinal stenosis, and degenerative disc disease, traditionally requires placement of 4 screws and 2 rods, creating an artificial bridge, to stabilize the segment.
- The conventional invasive approach requires precise placement and building of bridges intraoperatively.
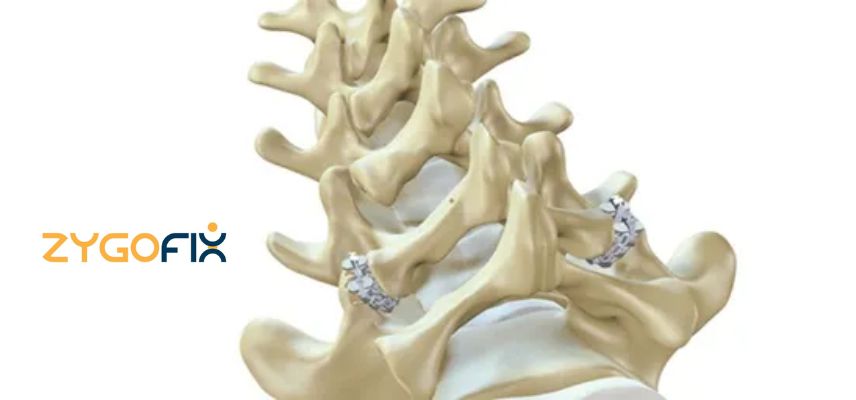
- ZygoFix's revolutionary zLOCK system harnesses the spine's innate bone structure to securely lock its connecting joints.
- Unlike traditional methods, the company's innovative implant adapts to the joint's anatomy during insertion, offering unparalleled stability with a minimally invasive, simple procedure that can be performed in outpatient and ASC environments.
With CE certification and six years of clinical utilization yielding long-term pain reduction and bone growth, ZygoFix's zLOCK Lumbar Facet Fixation System is positioned to revolutionize spinal fusion surgery.
Words from CEO: ZygoFix
ZygoFix CEO Ofer Levy, said: ""Receiving US FDA clearance for our zLOCK Lumbar Stabilization System is an exciting milestone, and I am very proud of the team that has worked hard to achieve this goal. The system has garnered significant interest in the US market and this clearance paves the way for us to introduce our transformative solution to the US market, fostering collaboration with leading surgeons and enhancing patient care.""
Dr. Nicholas Pachuda, ZygoFix Chair, added: ""Obtaining 510(k) clearance is a testament to ZygoFix's commitment to innovation and addressing the unmet needs of patients suffering from degenerative spinal conditions. We eagerly anticipate offering patients our minimally invasive, life-changing solution in the United States.""
From an Expert
Dr. Isador Lieberman, orthopedic & spine surgeon at Texas Back Institute, commented: ""The simplicity and minimally invasive nature of the zLOCK system provides a new tool in the surgeon's offering. For the right patients, zLOCK can provide a great alternative to pedicle screws."

Optimize Your trial insights with Clival Database.
Are you exhausted from the uncertainty of trial insights pricing? Clival Database ensures the clarity in the midst of the global scenario for clinical trials to you.Clival Database is one of the best databases that offers an outstanding number of clinical trial data in terms of 50,000+ molecules and from primary regulatory markets as well as new entrants like Indian and Chinese markets.
With Clival, you get accurate positioning of historical sales data, patent database, company profiling, safety & efficacy, and prediction of launch of new innovative molecules helping you to align your research and driving down the cost.
To add value, we further break down our analytics for you so that improving your operational effectiveness; optimizing your clinical trials; and offering you accurate and high-quality data at lowest possible prices becomes possible.
Elevate your trial success rate with the cutting-edge insights from Clival database.
Check it out today and make more informed sourcing decisions! Learn More!